October 5, 2021
Nine LED Brands vs. HID vs. the Sun

A recent study compares the performance of various horticultural lighting sources
A recently published study from researchers at Cornell University and Rutgers University emphasizes a substantial range of performance characteristics among horticultural light sources, highlighting the importance of a careful assessment before selecting a light source for horticultural applications. Some of the takeaways include:
- The Photosynthetic Photon Flux (PPF) efficacy of a light source is but one performance characteristic that should be considered.
- There are currently some imperfections, lack of consistency in the ways that various manufacturers report the performance characteristics of light sources, making it difficult for end users to do attribute comparisons.
- Due to the rapidly improving LED technology, it is critically important to have a consistent system for measuring and reporting lamp characteristics.
Below is a reprint of the study:
On the Technical Performance Characteristics of Horticultural Lamps
by Timothy J. Shelford, School of Integrative Plant Science, Horticulture Section, Cornell University and Arend-Jan Both, Department of Environmental Sciences, Rutgers University
1. Introduction
2. Materials and Methods
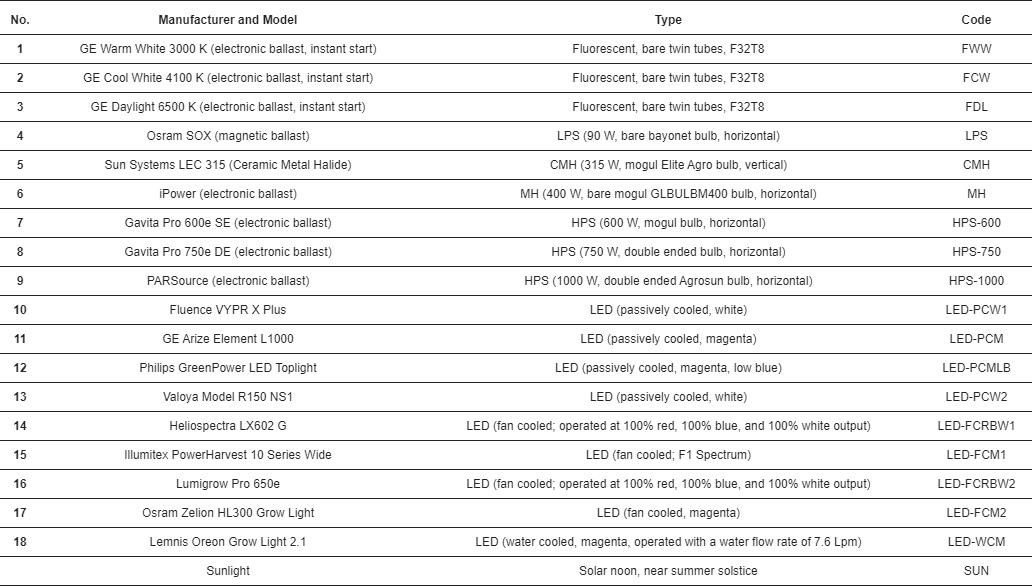
2.1. Light Sources
2.2. Measurement Equipment
2.3. Environmental Conditions
2.4. Calibration
2.5. Definitions and Calculations
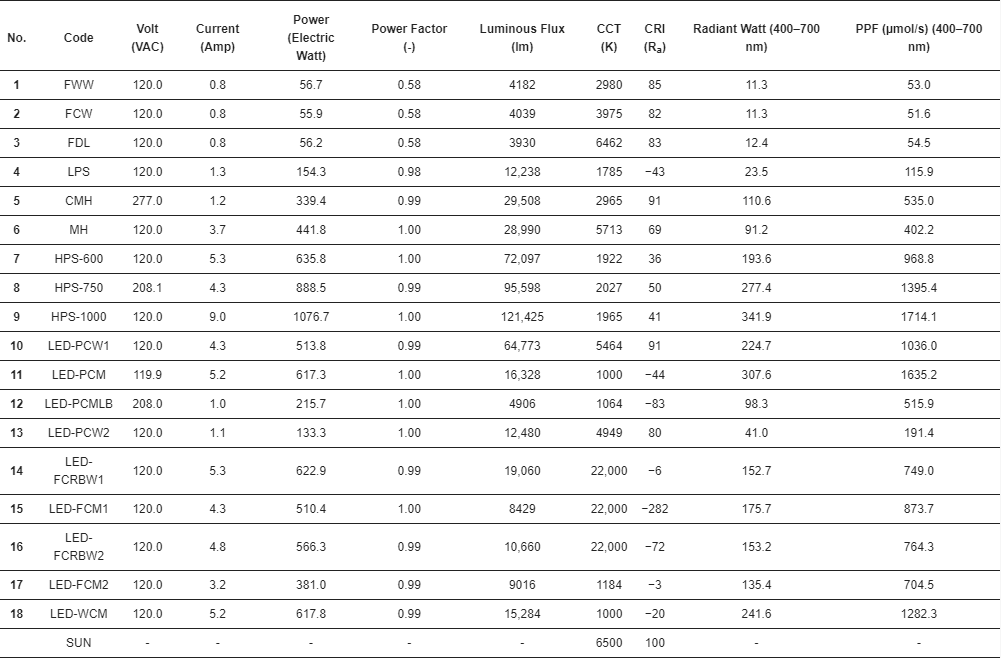
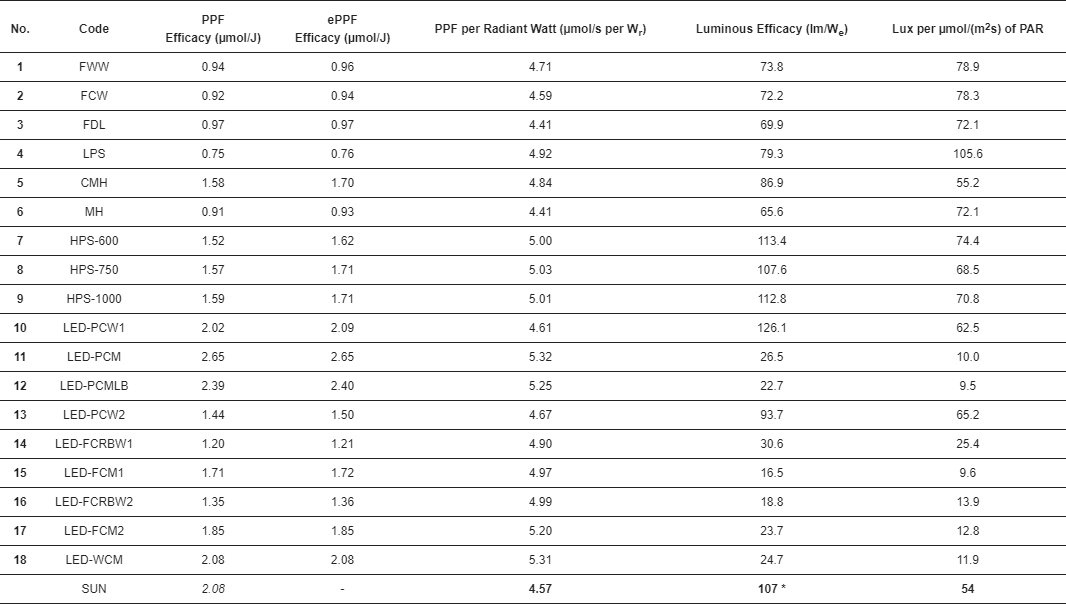
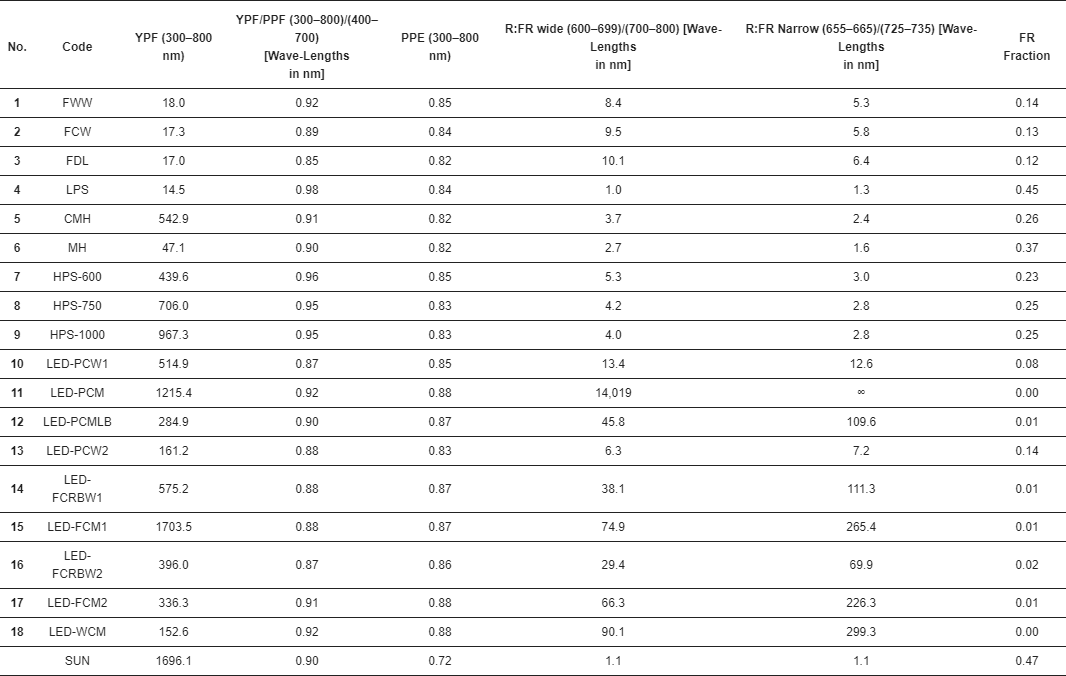
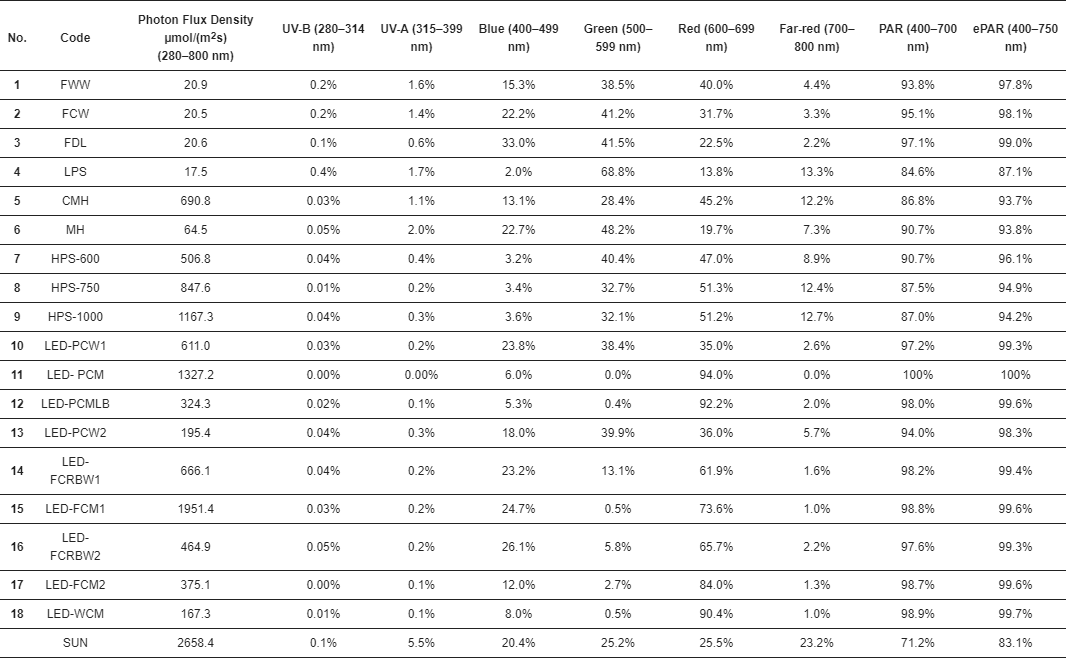
3. Results
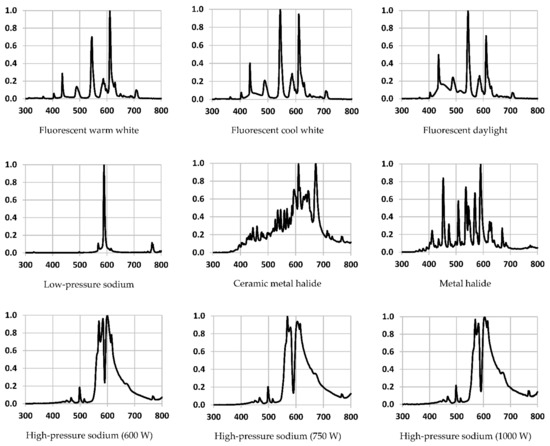
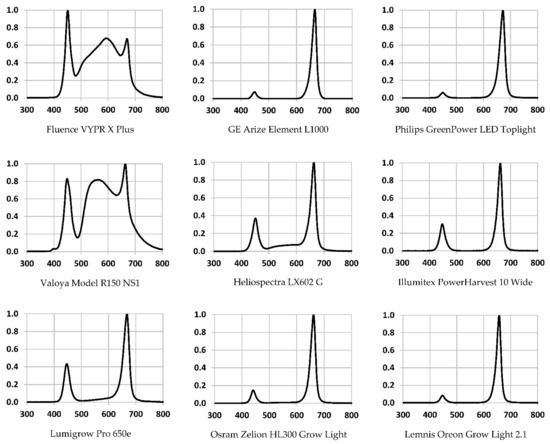
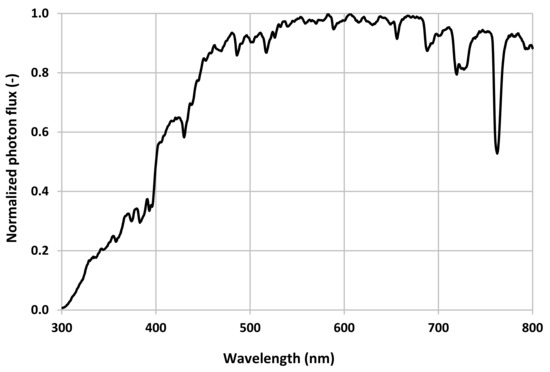
4. Discussion
5. Conclusions
-
Every light source tested had unique performance characteristics, including their spectral outputs.
-
The PPF efficacy of a light source is but one performance characteristic that should be considered.
-
A spectroradiometer is needed in order to assess the spectral output of a light source.
-
Changing the definition of PAR will make it more difficult to compare published results that used the current definition for PAR (400–700 nm) with results published based on the extended definition for PAR (ePAR, 400–750 nm).
-
The sooner the scientific community can agree on definitions that describe key performance characteristics (e.g., waveband ranges, photosynthetically active radiation), the less confusion there will be when these performance characteristics are used to make plant lighting decisions.
-
Due to the rapidly improving LED technology, it is critically important to have a consistent system for measuring and reporting lamp characteristics.
-
Due to the challenges involved, commercial growers are encouraged to experiment with new light sources on a small growing area, before deciding to scale up to large production areas.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, C.A.; Burr, J.F.; Dzakovich, M.J.; Gómez, C.; Lopez, R.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-Emitting Diodes in horticulture. In Horticultural Reviews; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–87. [Google Scholar]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Bugbee, B. Toward an optimal spectral quality for plant growth and development: The importance of radiation capture. Acta Hortic. 2016, 1134, 1–12. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef]
- Katzin, D.; Marcelis, L.F.M.; van Mourik, S. Energy savings in greenhouses by transition from high-pressure sodium to LED lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
- Lopez, R.; Runkle, E.S. (Eds.) Light Management in Controlled Environments; Meister Media: Willoughby, OH, USA, 2017; 180p. [Google Scholar]
- Mickens, M.A.; Skoog, E.J.; Reese, L.E.; Barnwell, P.L.; Spencer, L.E.; Massa, G.D.; Wheeler, R.M. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.; Both, A.J. Evaluating operating characteristics of light sources for horticultural applications. Acta Hortic. 2016, 1134, 435–444. [Google Scholar] [CrossRef]
- Both, A.J.; Bugbee, B.; Kubota, C.; Lopez, R.G.; Mitchell, C.; Runkle, E.S.; Wallace, C. Proposed product label for electric lamps used in the plant sciences. Hort. Technol. 2017, 27, 544–549. [Google Scholar] [CrossRef]
- Shelford, T.; Wallace, C.; Both, A.J. Calculating and reporting key light ratios for plant research. Acta Hortic. 2020, 1296, 559–566. [Google Scholar] [CrossRef]
- Thimijan, R.W.; Heins, R.D. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. HortScience 1983, 18, 818–822. [Google Scholar]
- ANSI/ASABE Standard S640. Quantities and Units of Electromagnetic Radiation for Plants (Photosynthetic Organisms); American Society for Agricultural and Biological Engineers: St. Joseph, MI, USA, 2017. [Google Scholar]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.; Bugbee, B. Why far-red photons should be included in the definition of photosynthetic photons and the measurement of horticultural fixture efficacy. Front. Plant Sci. 2021, 12, 693445. [Google Scholar] [CrossRef] [PubMed]
- Pazuki, A.; Aflaki, F.; Pessarakli, M.; Gurel, E.; Gurel, S. Plant responses to extended photosynthetically active radiation (EPAR). Adv. Plants Agric. Res. 2017, 7, 00260. [Google Scholar]
- Barnes, C.; Tibbitts, T.; Sager, J.; Deitzer, G.; Bubenheim, D.; Koener, G.; Bugbee, B. Accuracy of quantum sensors measuring yield photon flux and photosynthetic photon flux. HortScience 1993, 28, 1197–1200. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Transact. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. Improving the predictive value of phytochrome photoequilibrium: Consideration of spectral distortion within a leaf. Front. Plant Sci. 2021, 12, 596943. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, P.; Bugbee, B. Far-red fraction: An improved metric for characterizing phytochrome effects on morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
- Radetsky, L.C. LED and HID Horticultural Luminaire Testing Report; Lighting Research Center, Rensselaer Polytechnic Institute: Troy, NY, USA, 2018; 80p. [Google Scholar]
- Ting, K.C.; Giacomelli, G.A. Availability of solar photosynthetically active radiation. Transact. ASAE 1987, 30, 1453–1457. [Google Scholar] [CrossRef]
Reprinted from: Advanced Lighting Technologies in Controlled Environment Agriculture for Production, Breeding, and Research
Don’t miss the next big lighting story…Click here to subscribe to the inside.lighting InfoLetter |